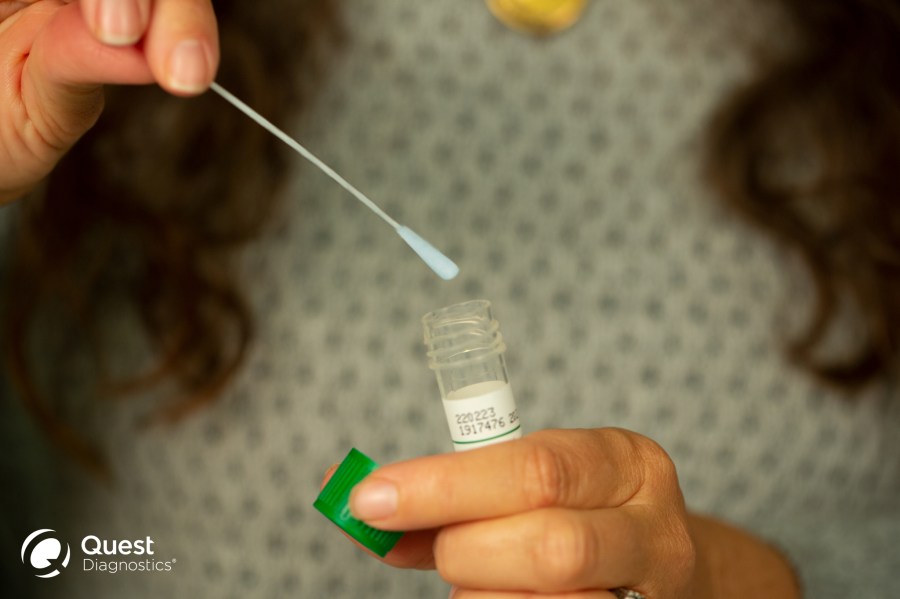
SECAUCUS, NJ (WBTW) – Quest Diagnostics announced Thursday permission from the FDA to use their COVID-19 self-collection kit for individuals to self-collect a nasal specimen at home or in a healthcare setting when determined to be appropriate by a healthcare provider.
The self-collection kit allows an individual to swab the front part of the nostril and may be used on children (supervised by an adult) as well as adults. Specimens are shipped overnight via FedEx at room temperature (without a frozen cold pack).
“COVID-19 molecular diagnostic testing has been constrained partly by limited supplies of swabs and trained healthcare professionals to do the specimen collection,” said Steve Rusckowski, Chairman, CEO and President. “The self-collection kit enables an individual to self-collect at home, and the process is far less invasive and uncomfortable than many traditional methods.”
Specimens collected using the kit may be tested with the Quest Diagnostics test that received an Emergency Use Authorization in March. This testing aids in diagnosing infection with the virus that causes COVID-19.
“We plan to utilize this device with a range of populations, from state-run programs and employers, to healthcare providers and individuals,” said Jay G. Wohlgemuth, M.D., Senior Vice President and Chief Medical Officer. “Our scientists at our advanced diagnostics laboratory in San Juan Capistrano, California developed the technology, which has been validated in real-world studies.”
Quest shared data with FDA that indicates the self-collection kit offers a consumer-friendly approach to high quality diagnostic testing for COVID-19. Quest Diagnostics already tested specimens using a similar collection method in real-world settings in drive-thru and other onsite COVID-19 testing sites across the United States.
The self-collection kit was developed to be very consumer friendly, with the specimen collected at home and without the need to directly involve a healthcare professional to perform or observe the collection.
Key features of the new kit include:
- Self-collection by individuals, at home, with a consumer-friendly nasal swab approach
- Overnight shipping to the individual and back to Quest Diagnostics with FedEx, leveraging their extensive logistics network
- Specimens shipped at room temperature, which eliminates the need for ice packs
- Availability for children less than 18 years of age (with adult supervision)
- Results reporting through the myQuest patient portal and mobile app
- Test data reported by Quest Diagnostics to the relevant departments of health as required
The company plans to make the self-collection kits available through several channels, including for healthcare providers for patient care and healthcare workers, as well as for states and organizations for return-to-work testing programs. Over time, the kits may also be made available to other employers as well as for individual users of the company’s QuestDirect consumer-initiated platform.
The company will prioritize healthcare workers, first responders, law enforcement personnel and others critical to pandemic response to ensure they have timely access to the kit.
The company expects to have more than a half-million kits available by the end of June, with plans to make additional kits available on an ongoing basis.
For more information about the latest developments with the company’s COVID-19 testing, visit:
newsroom.questdiagnostics.com/COVIDTestingUpdates
LATEST HEADLINES